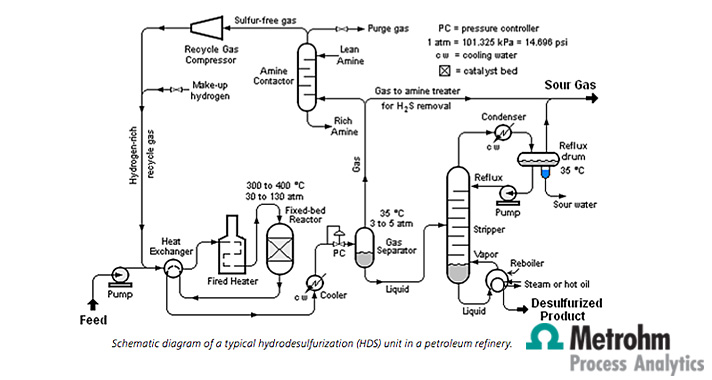
Schematic diagram of a typical hydrodesulfurization (HDS) unit in a petroleum refinery.
Mercaptans (thiols) and hydrogen sulfide (H2S)
Fossil fuels are known for their sulfur content, which originates from the decomposition of dead organisms over millennia. Mercaptans (thiols) and hydrogen sulfide (H2S) are two sulfur compounds present in crude oil which contribute to its characteristic odor. In the refining process, these can lead to increased corrosion in distillation equipment at the high temperatures used. Additionally, excess sulfur dioxide (SO2(g), a pollutant) can be emitted after combustion if sulfur is present in the refined products. Therefore, it is in our best interest to remove as much sulfur as possible early in the refining process.
Sulfur compounds are present throughout the entire boiling range of hydrocarbons in crude oil. Depending on the size and bond strength of these compounds, different desulfurization treatments are available. Lighter impurities (including mercaptans and sulfides) can be removed via hydrotreating in a reactor with a catalyst (generally cobalt molybdenum) and hydrogen under high temperature and pressure. Heavier sulfur compounds can be removed with hydrocracking after the hydrotreating process. Desulfurization can occur at many points within a refinery, from the crude feedstock to the distillate streams. A Metrohm Process Analytics 2045TI Ex-proof Process Analyzer with a customized sample preconditioning system could be implemented in many areas within a refinery to ensure the catalysts in the reactors are not exhausted and to limit corrosion further on in the distillation unit. The analyzer fulfills EU Directives 94/9/EC (ATEX95) and is certified for Zone-1 and Zone-2 areas.