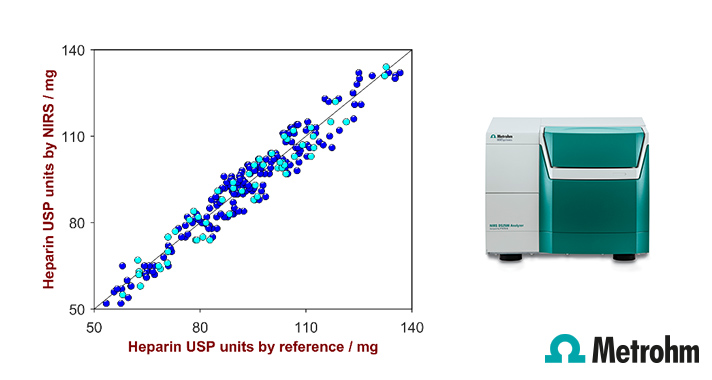
Correlation plot of the predicted heparin sodium product strength by NIRS versus the laboratory values. Displayed are the training data (blue) and validation data (turquois).
Heparin
Heparin is a glycosaminoglycan used as an anticoagulant for therapeutic uses. It can either be used directly for humans given by injection or used as a lock flush solution to maintain the patency of intravenous injection devices (IID). The U.S. Pharmacopeial Convention (USP) regulates the product strength of heparin sodium doses as well as the heparin lock flush solution.
Strength determination
The product strength of heparin sodium has to be determined accurately and precise to guarantee the desired physiological effect on patients as well as an effective maintenance of intravenous injection devices. This determination is run using UV-Vis spectroscopy needing multiple reagents and takes up a great deal of time. A reduction of time expense and reagents should be strived for. This can be accomplished using near-infrared spectroscopy.
Applicability of Vis-NIR spectroscopy
Metrohm’s application note AN-NIR-042 demonstrates the applicability of Vis-NIR spectroscopy to determine the product strength of heparin sodium precipitate.