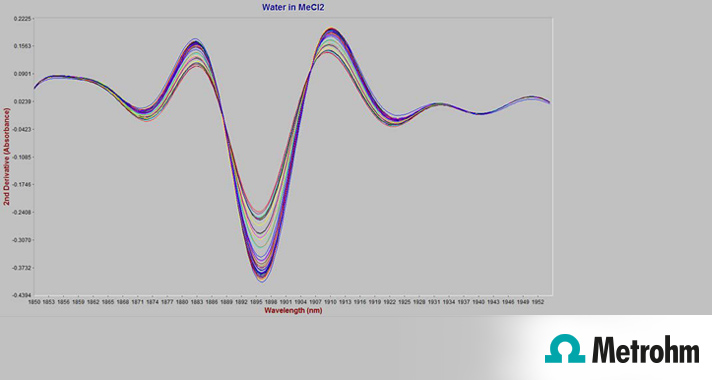
The purity of a solvent (dichloromethane/methylene chloride) is monitored by NIR spectroscopy along with two of the major impurities (methanol and water).
Recovering solvents
The solvents that are used in manufacturing processes are often not disposed of or incinerated, but instead recovered and purified, as this saves considerable costs.
Distillation
Used solvents are mostly purified by distillation. Solvent recovery processes are very common in the chemical industry and in the pharmaceutical industry in the manufacture of APIs (Active Pharmaceutical Ingredients).
Advantages of NIR
Near-Infrared (NIR) spectroscopy can be used to ensure that the solvents are sufficiently pure for reuse in manufacturing. It requires no sample preparation and produces results in a matter of seconds. Furthermore, it does not require trained analysts, and it can be performed as a lab method using grab samples or directly inline.
Summary
NIR spectroscopy can be used to analyze the concentration of several impurities in recovered solvents and estimate their overall purity. Multiple analyses can be done simultaneously in less than 30 seconds total analysis time. While this study was done using a NIRS XDS SmartProbe Analyzer in a laboratory environment, the application could also be done with a NIRS XDS Rapid Liquid Analyzer – using cuvettes or disposable vials. Alternatively, these analyses can be done directly in the distillation process (liquid output stream of the distillation column) with a fiber-optic-based NIRS XDS Process Analyzer. In the latter case, the entire distillation process can be monitored and controlled by the NIR analyzer.
Download the complete Metrohm Application Note NIR–21 below for more details or contact us directly !