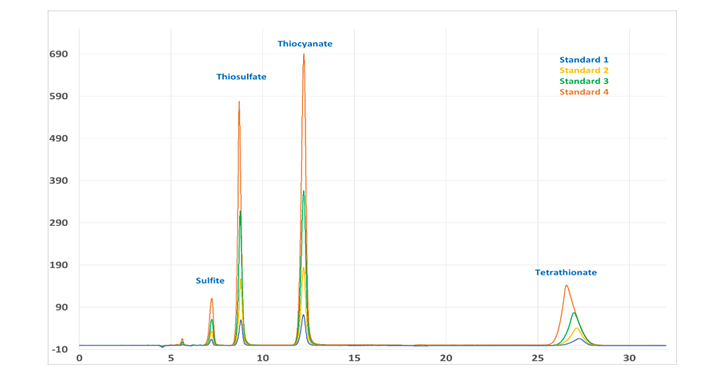
This application has been developed to determine all the common sulfur species, in exception of sulfate, via ion exchange chromatography. The aims of the application development have been achieved using perchlorate as a mobile phase and utilizing direct UV absorption as detection technique.
Substitute to cyanide leaching processes
Health and safety pressures are motivating the gold mining industry to develop a substitute to cyanide leaching processes. The drive is mainly based on the high toxicity of cyanide.
The chemistry involved in the thiosulfate leaching process is more complex and less robust than the cyanide leaching process. It is, therefore more difficult to optimize and more sensitive to operate. The leach process involves a chemical reaction between metallic gold and the thiosulfate anion with oxygen as the oxidant, ammonium and copper ions are unconsumed catalysts in the reaction.
Thiosulfate forms strong complexes with Au(I), Cu(I), Cd(II), Bi(II), Hg(II) and Fe(III). In the presence of ammonium thiosulfate with oxygen, gold complexes with thiosulfate as follows:
4Au + 8S2O32– + O2 + 2H2O → 4 Au(S2O3)2 3– + 4OH–
While it is relatively easy to establish and optimize the amenability of an ore to a cyanide leaching process, the thiosulphate chemistry presents technical challenges which require careful optimization. Thiosulphate leaching is a sensitive process that requires both dependent and independent optimization of each of the chemical components of the leach reaction in order to maximize gold recovery and minimize reagent losses.
Sulfur speciation
This application has been developed to determine all the common sulfur species, in exception of sulfate, via ion exchange chromatography. The aims of the application development have been achieved using perchlorate as a mobile phase and utilizing direct UV absorption as detection technique.
Perchlorate, as a mobile phase, allow analysis, without excessive sample preparation, of samples that may contain relative high concentrations of transition and heavy metals.
Referring to the table below, most of the metal perchlorates are highly soluble in water in comparison to the respective metal hydroxides. This confirms the choice of perchlorate as a mobile phase in order to diminish potential precipitation on the anion exchange column.