The Metrohm Raman Mira M-3 is fully compliant with FDA 21 CFR Part 11 providing multilevel access control, audit trails, secure electronic records and other features to exceed regulatory requirements.
Onsite verification of incoming raw materials in seconds.
Handheld Raman spectrometer is now widely used for applications in the pharmaceutical industry. Within the pharmaceutical industry, one of the biggest applications of handheld Raman spectrometers are the identification of incoming raw materials and finished product inspection. The other major application is the analysis of polymorphs and salt forms in both quality control (QC) and product development, variations of which can lead to dramatically different performance of a pharmaceutical product.
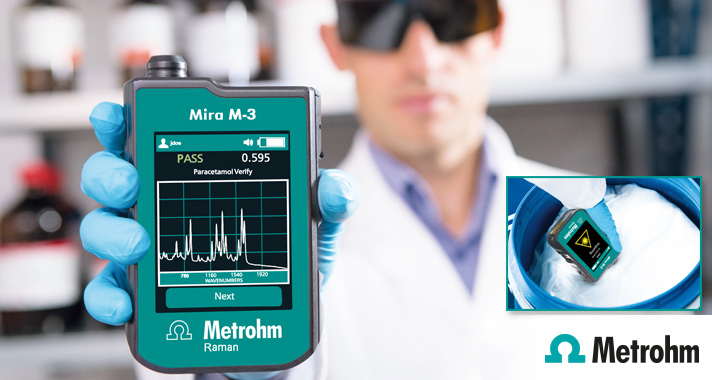
The Metrohm Instant Raman Analyzer (MIRA) M-3 is a high performance, handheld Raman spectrometer used for rapid, non-destructive identification and verification of different material types, such as incoming raw materials, Pharmaceutical APIs, excipients etc. Despite the small size of the instrument, the M-3 has a ruggedized design and features a high-efficiency spectrograph design equipped with our unique Orbital-Raster-Scan (ORS) technology. The Mira M-3 is fully compliant with FDA 21 CFR Part 11 regulations.
Operating Procedures can be customized on the Mira M-3 to allow sampling flexibility. Routine users start their measurements at the push of a button to verify the identity of materials with a pass/fail result in a few seconds.
Dedicated sampling attachments such as a Vial Holder (for powders and liquids) and a Tablet Holder (for tablets and pills) are part of the Mira M-3 and can be used without any further laser protection (Laser Safety Class 1). Point-and-shoot attachments are also available for contact measurements through barriers (e. g., glass bottles).
The Mira M-3 is fully compliant with FDA 21 CFR Part 11 providing multilevel access control, audit trails, secure electronic records and other features to exceed regulatory requirements.