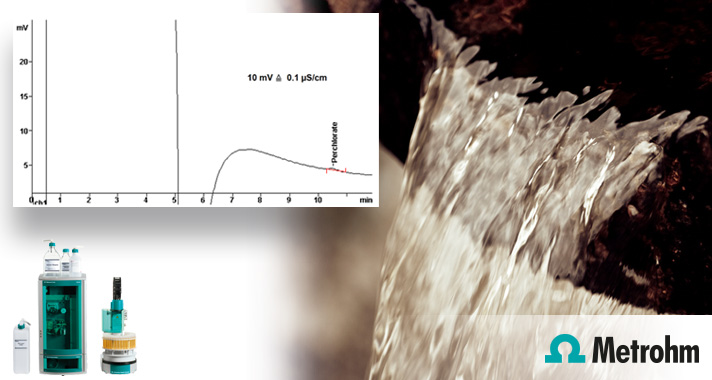
The figure showing the results of the determination method: Perchlorate in 3000 ppm TDS L : 0.5 μg/L Lowest concentration minimum reporting level (EPA-LCMRL required: 0.57 μg/L) : 0.11 μg/L
Perchlorate (ClO4–), is a manufactured chemical used as the main ingredient of rocket fuel, safety flares, matches and fireworks. It is also used in some batteries, and vehicle air bags. Chemically, perchlorate is an anion that originates from the dissolution of ammonium, potassium, magnesium, or sodium salts. It is very mobile in ground water and surface water systems and can persist for many decades. Perchlorate travels with water and unlike some contaminants, doesn’t stick to surfaces within the soil or aquifers. Perchlorate contamination of ground water only became widely known in the late 1990’s when new laboratory techniques lowered the detection limit in water from 400 to 4 micrograms/L.
The principal health concern is that if perchlorate gets into drinking water it could damage the thyroid gland, which controls growth, development and metabolism. Perchlorate interferes with iodide uptake into the thyroid gland, and because iodide is an essential component of thyroid hormones, it disrupts how the thyroid functions. Impairment of a woman’s thyroid function during pregnancy may impact the baby and result in delayed development and decreased learning capability. Changes in thyroid hormone levels may also result in thyroid gland tumors. Determining the threshold levels of ingested perchlorate needed to inhibit the uptake of iodide, is the subject of ongoing medical research.
Metrohm has developed a method to determine perchlorate with ion chromatography with suppressed conductivity detection down to 0.11 ppb in a high ionic strength matrix.