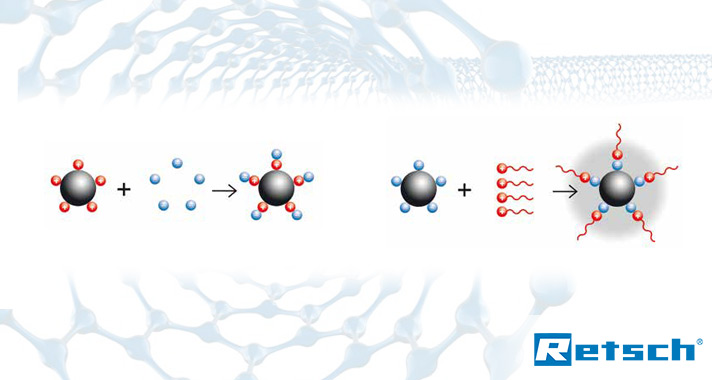
Neutralization of charged particles by adding a buffer (electrostatic stabilization, left) or by adding long-chained molecules (steric stabilization, right) – Factors such as energy input and size reduction principle make ball mills the best choice for the production of nanoparticles.
From 1 to 100 nm
Nanotechnology is one of the most innovative developments of our time which revolutionizes industries such as materials science, pharmaceutics, food, pigments or semi-conductor technology.
Nanotechnology deals with particles in a range from 1 to 100 nm. These particles possess special properties due to their size, as their surface is greatly enlarged in relation to their volume (so-called “size-induced functionalities”).
Ultrafine particles are, for example, harder and more break-resistant than larger particles.
Nanotechnology brings effects which occur in nature to a commercial scale, such as, for example, the lotus effect: nano-coated fabrics or paints are water- and dirt-repellent just like the lotus flower.
How are nano particles produced?
The “Bottom-Up” method synthesizes particles from atoms or molecules.
The “Top-Down” method involves reducing the size of larger particles to nanoscale, for example with laboratory mills.
Due to their significantly enlarged surface in relation to the volume, small particles are drawn to each other by their electrostatic charges.
Nano particles are produced by colloidal grinding which involves dispersion of the particles in liquid to neutralize the surface charges. Both water and alcohol can be used as dispersion medium, depending on the sample material. In some cases the neutralization of surface charges is only possible by adding a buffer such as sodium phosphate or molecules with longer uncharged tails such as diaminopimelic acid (electrostatic or steric stabilization).